National clinical study
OPTIMISE - OPTIMIzation of treatment SElection and follow up in oligometastatic colorectal cancer - a ctDNA guided phase II randomized approach
A study investigating if analysis of circulating tumor DNA (ctDNA) can guide adjuvant treatment in patients with advanced colorectal cancer (CRC). An open label 1:1 randomized phase II exploratory study investigating use of ctDNA-guided adjuvant chemotherapy compared to standard of care (SOC) after local treatment for metastatic colorectal cancer.
ClinicalTrials.gov: NCT04680260
The project received funding in 2020 and 2023.
Principal Investigator (PI)

Study Coordinator

Collaborators
Sygehus Lillebælt, Vejle Hospital
Aarhus University Hospital
Patient enrollment
350
Cancer
Oligometastatic colorectal cancer
Type
Randomised interventional
Platform
ddPCR
MassARRAY
Abstract
Perspectives: Oligometastatic colorectal cancer can be cured with local treatment, but with a high risk of recurrence. Currently, clinical indicators for either early detection of recurrence or optimal selection of post-treatment chemotherapy are missing. In the present study, we therefore investigate circulating tumour DNA (ctDNA)-guided treatment in the oligometastatic setting to optimize use of chemotherapy and hereby outcome.
Background: In Denmark approximately 5,000 patients are diagnosed with colorectal cancer each year, 50% will eventually develop metastatic spread, with an overall poor prognosis. There is a curative potential from treating single or few local metastases when sited in the liver or lung. Some patients obtain long-term survival, whereas others show an aggressive biological behaviour with early recurrence and systemic dissemination despite the use of adjuvant chemotherapy. At present, there is no clear consensus on the use of chemotherapy in relation to local treatment in oligometastatic colorectal cancer, due to limited evidence.
Presence of ctDNA in the plasma post-surgery for primary colorectal tumours indicates microscopic residual disease and a subsequent very poor outcome. This is demonstrated in a Danish pilot study in the oligometastatic setting (Boysen et al. 2019). It is consequently highly relevant to initiate a clinical trial of ctDNA-guided post-treatment strategy in the oligometastatic setting. Such strategy must be evaluated against current standard of care to investigate the true clinical utility, and for that purpose feasibility measures need to be established prospectively. Consequently, we will launch a feasibility run-in study in two oncology centres before expanding into a planned larger phase II randomized multicentre study.
Aim: The primary aim of the present study is to investigate – in a randomized trial – the clinical utility of ctDNA analysis to guide treatment decisions in oligometastatic colorectal cancer. Secondary aims include investigating molecular biological response to chemotherapy, cost effectiveness, and quality of life.
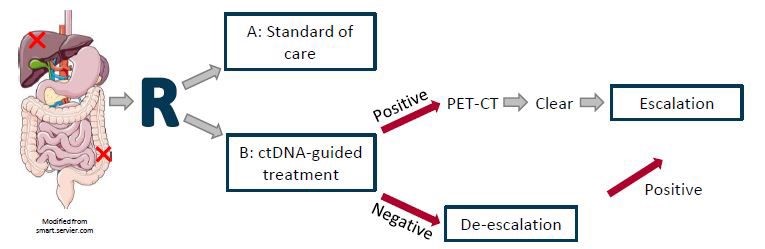
Methods: An open label 1:1 randomized phase II exploratory study investigating use of ctDNA-guided therapy compared to standard of care after local treatment for metastatic CRC. Circulating cell free DNA will be analysed for known CRC specific mutations by digital droplet PCR panel and for hypermethylation by methylation assay. ctDNA positivity will lead to escalation of chemotherapy consisting of 4 months of FOLFOXIRI followed by 2 months of 5FU monotherapy. ctDNA negativity will based on shared decision-making lead to de-escalation i.e. less or no chemotherapy. The study will run in two Danish centres (AUH and Vejle) and be expanded if feasibility criteria are met.
Expected outcome: This study will demonstrate the feasibility of patient inclusion, rate of imaging detected residual disease, rate of ctDNA positivity and shared decision making in ctDNA-guided therapy for oligometastatic colorectal cancer. This will lead to a larger multicentre, phase II randomized study, investigating the clinical utility of ctDNA-guided treatment in oligometastatic colorectal cancer.
Study design
ADDRESS FOR THE SECRETARIAT
Science Center Skejby, MOMA
Brendstrupgårdsvej 21, build. A
8200 Aarhus N
CONTACT
ctDNA@clin.au.dk
+45 78 45 53 39

